How To Know Chemical Compound Makeup Given Weight Precentage
Summate mass, concentration and pH of solutions
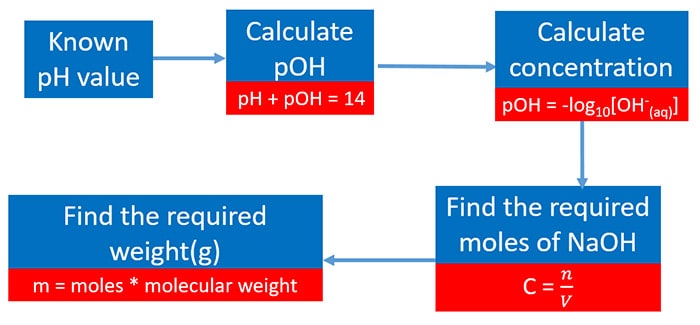
In this tutorial, we are going to do some bug which contains relationship of mass, concentration and pH in solutions. You can empathise how to apply those equations in such problems to practise these type of questions easily.
Question i
Summate the NaOH weight required to prepare 500ml of NaOH solution of pH=13. Room temperature is 250C.
Calculate concentration of solution of known pH
Calculate pOH from pH by using the pH + pOH = xiv (at 250C)
Then you can calculate the concentration of NaOH solution by pOH = -log10[OH- (aq)] .
So find the required moles of NaOH past the equation of C =due north/v . Here C = concentration, n=required moles, five = volume of solution
Now weight is measured by multiplying number of moles and molar mass.
Assumptions
The OH- received from water dissociation is negligible when information technology compares with OH- received from NaOH.
Calculate pOH from pH value
pH + pOH = 14
13 + pOH = 14
pOH = 1
Calculate concentration (in mol dm-3) of solution
Now nosotros know the pOH of NaOH solution. Employ the relation of pOH and OH- concentration to summate the concentration of OH-.
pOH = -log10[OH- (aq)]
1 = -log10[OH- (aq)]
[OH- (aq)] = 0.1 mol dm-3
Discover the required moles of NaOH
Concentration, moles amount, and volume relation is used.
C = n/five
0.1 = northward/0.five [500 ml = 0.v dm3]
n = 0.05mol
NaOH amount = 0.05 mol
Find the required weight(g)
Multiply required moles times molecular weight
m = mass
northward = moles
G = molecular weight
n = one thousand/M
m = molecular weight * moles
m = Thou*n
m = 0.05 * forty
m = 2g
How to summate mass given ph and volume of a solution?
Ok. First we should know, what are the known parameters of the given solution. In this instance, pH and volume are given as data.
When pH value is known, concentration of HthreeO+ or OH- can be foundfrom pH or pOH equation.
According to the H3O+ or OH- concentration and stoichiometric ratio of dissociation of the chemical compound, the concentration of dissolved compound is found.
With plant concentration and volume of solution, dissolved amount is found.
Amount = concentration * volume
Next, nosotros use human relationship of corporeality, mass and molecular mass.
Amount = mass / molecular mass
Because nosotros know the amount and molecular mass, dissolved mass tin be institute.
Related tutorials
How To Know Chemical Compound Makeup Given Weight Precentage,
Source: https://www.chemistryscl.com/advancedlevel/physical/calculate-weight-concen-ph/index.php
Posted by: furnesswidefirearm.blogspot.com


0 Response to "How To Know Chemical Compound Makeup Given Weight Precentage"
Post a Comment